Microsoft word - learning to read.doc
Learning To Read without a Teacher: A Case Study* This chapter is the story of John, middle child in a black family of five, son of a trucker and a hospital maid, living in subsidized housing in a crowded southern city. According to most predictions, John should have reading problems all through his school career. But John is one of the thousands of children who somehow learn to read in mu
 nent microporosity even in the absence of guests, an aspect
that is routinely considered for zeolites[2] but has remained
largely unexplored for the analogous metal ± organic materi-
als.[3] In attempting to address this issue, we aimed at coupling
our interest in designing new frameworks with the desire to
achieve stable microporous structures. Here we report the
synthesis and structure of Tb(bdc)NO3 ´ 2DMF (bdc 1,4-
benzenedicarboxylate; DMF N,N-dimethylformamide) and
show that its desolvated derivative Tb(bdc)NO3 has a stable
zeolite-like framework that is capable of reversible molecular
sorption and of maintaining microporosity in the absence of
Previous studies on the copolymerization of ZnII with BDC
have shown that stable frameworks can be produced.[3d, 4] This
was attributed to the bis-bidentate functionality of BDC and
its tendency to form large, tightly bound metal carboxylate
cluster aggregates that ultimately act as building blocks in the
crystal structure. We sought to extend this strategy to the
pursuit of lanthanide ± organic open frameworks, which
remain virtually unknown, despite the established role of
lanthanide compounds sensor technology.[5]
Deprotonation of the acid form of BDC (H2BDC) with
pyridine followed by its copolymerization with TbIII in
methanol/DMF at room temperature gave a crystalline color-
less solid, which was formulated as Tb(bdc)NO3 ´ 2DMF on
the basis of elemental analysis and single-crystal X-ray
diffraction.[6, 7] Complete deprotonation of BDC was con-
firmed by the absence of any strong absorption bands due to
protonated carboxyl groups (1715 ± 1680 cmÀ1) in the FT-IR
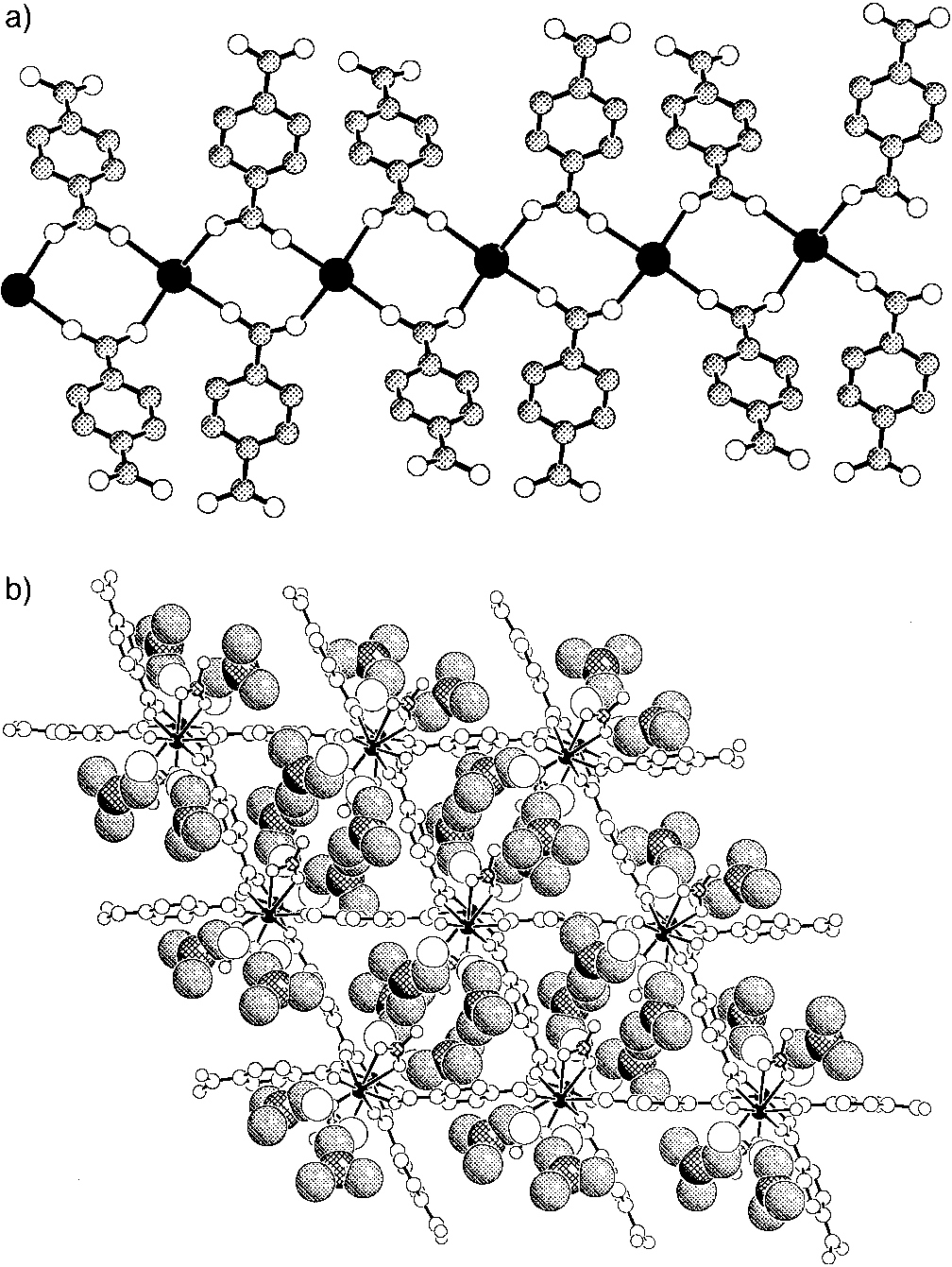
Figure 2. a) Tb ± BDC chains shown perpendicular to the c axis. b) A
spectrum.[6] This material is stable in air and is insoluble in
projection along the c axis with DMF shown in space-filling (C, shaded; N,
common organic solvents such as methanol, ethanol, acetoni-
cross-hatched; O, open) and the Tb ± BDC ± NO3 framework as ball-and-
stick (Tb, filled; N, cross-hatched; C and O, open) representations.
nent microporosity even in the absence of guests, an aspect
that is routinely considered for zeolites[2] but has remained
largely unexplored for the analogous metal ± organic materi-
als.[3] In attempting to address this issue, we aimed at coupling
our interest in designing new frameworks with the desire to
achieve stable microporous structures. Here we report the
synthesis and structure of Tb(bdc)NO3 ´ 2DMF (bdc 1,4-
benzenedicarboxylate; DMF N,N-dimethylformamide) and
show that its desolvated derivative Tb(bdc)NO3 has a stable
zeolite-like framework that is capable of reversible molecular
sorption and of maintaining microporosity in the absence of
Previous studies on the copolymerization of ZnII with BDC
have shown that stable frameworks can be produced.[3d, 4] This
was attributed to the bis-bidentate functionality of BDC and
its tendency to form large, tightly bound metal carboxylate
cluster aggregates that ultimately act as building blocks in the
crystal structure. We sought to extend this strategy to the
pursuit of lanthanide ± organic open frameworks, which
remain virtually unknown, despite the established role of
lanthanide compounds sensor technology.[5]
Deprotonation of the acid form of BDC (H2BDC) with
pyridine followed by its copolymerization with TbIII in
methanol/DMF at room temperature gave a crystalline color-
less solid, which was formulated as Tb(bdc)NO3 ´ 2DMF on
the basis of elemental analysis and single-crystal X-ray
diffraction.[6, 7] Complete deprotonation of BDC was con-
firmed by the absence of any strong absorption bands due to
protonated carboxyl groups (1715 ± 1680 cmÀ1) in the FT-IR
Figure 2. a) Tb ± BDC chains shown perpendicular to the c axis. b) A
spectrum.[6] This material is stable in air and is insoluble in
projection along the c axis with DMF shown in space-filling (C, shaded; N,
common organic solvents such as methanol, ethanol, acetoni-
cross-hatched; O, open) and the Tb ± BDC ± NO3 framework as ball-and-
stick (Tb, filled; N, cross-hatched; C and O, open) representations.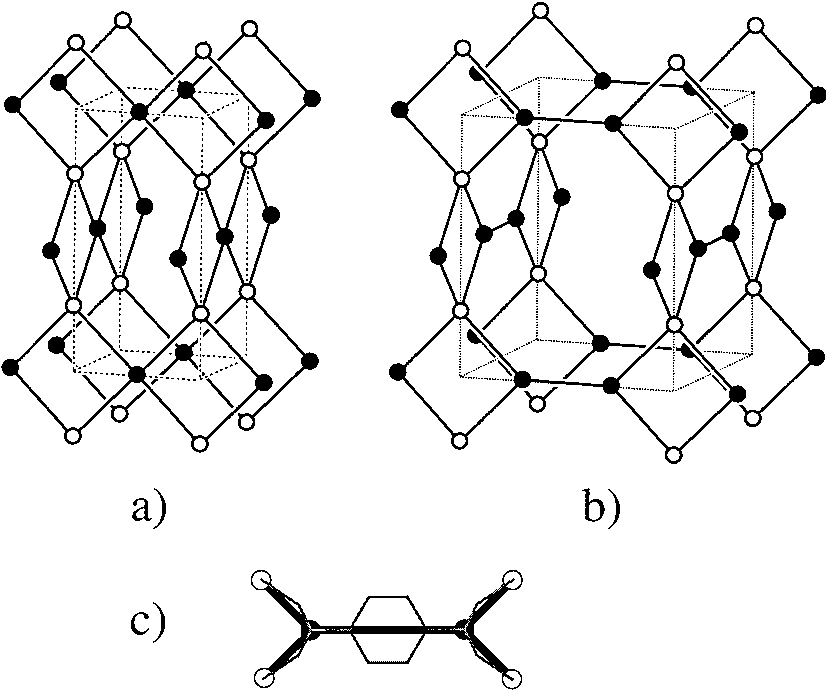
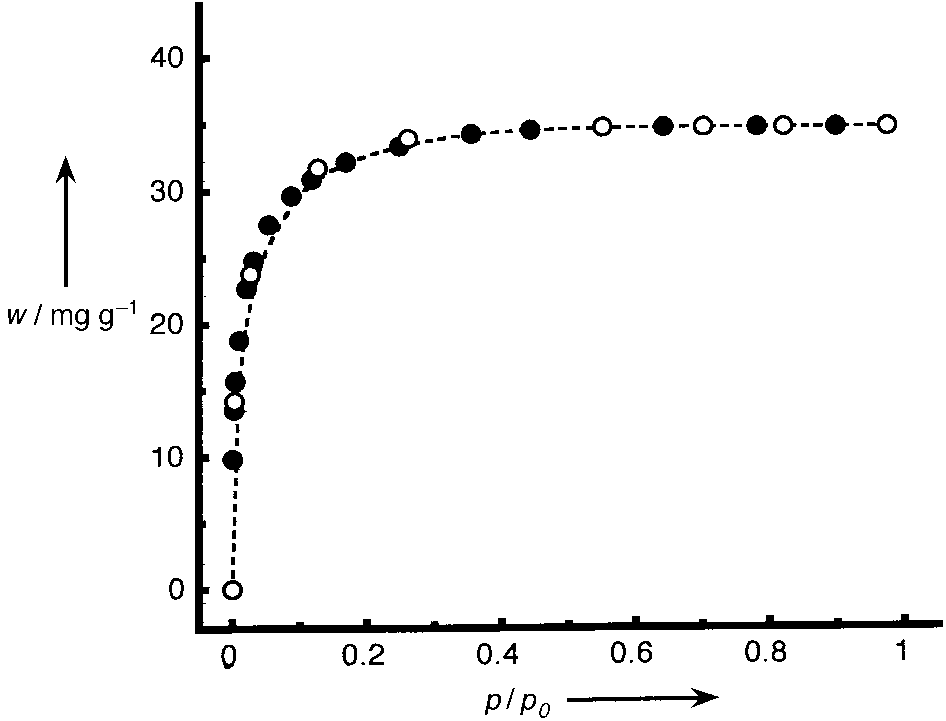 195 K. When no further weight change was observed, a single
isotherm point was recorded. A plot of weight sorbed per
gram of Tb(bdc)NO3 versus p/p0 (p0 saturation pressure;
780 Torr for CO2) revealed a reversible type I isotherm
(Figure 4), characteristic of a microporous material with
Figure 3. a) The 4-connected net of PtS (S, open; Pt, filled). b) The (3,4)-
connected net of Tb(bdc)NO3 ´ 2DMF that is derived from (a) by
converting the planar 4-connected vertices (filled) to pairs of 3-connected
vertices. c) Schematic identification of the atoms in the crystal structure of
Tb(bdc)NO3 ´ 2DMF with net vertices; open circles are Tb atoms [4-
connected vertices in (b)] and filled circles are carboxylate C atoms of
BDC [3-connected vertices in (b)]; the benzene ring of BDC is super-
imposed on the link between 3-connected vertices.
195 K. When no further weight change was observed, a single
isotherm point was recorded. A plot of weight sorbed per
gram of Tb(bdc)NO3 versus p/p0 (p0 saturation pressure;
780 Torr for CO2) revealed a reversible type I isotherm
(Figure 4), characteristic of a microporous material with
Figure 3. a) The 4-connected net of PtS (S, open; Pt, filled). b) The (3,4)-
connected net of Tb(bdc)NO3 ´ 2DMF that is derived from (a) by
converting the planar 4-connected vertices (filled) to pairs of 3-connected
vertices. c) Schematic identification of the atoms in the crystal structure of
Tb(bdc)NO3 ´ 2DMF with net vertices; open circles are Tb atoms [4-
connected vertices in (b)] and filled circles are carboxylate C atoms of
BDC [3-connected vertices in (b)]; the benzene ring of BDC is super-
imposed on the link between 3-connected vertices. Hoskins, J. Liu in Supramolecular Architecture: Synthetic Control in
Thin Films and Solids (Ed.: T. Bein), American Chemical Society,
Washington, DC, 1992, chap. 19; k) T. Iwamoto in Inclusion Com-
pounds, Vol. 5 (Eds.: J. L. Atwood, J. Davies, D. D. MacNicol), Oxford
University Press, New York, 1991, p.177.
Hoskins, J. Liu in Supramolecular Architecture: Synthetic Control in
Thin Films and Solids (Ed.: T. Bein), American Chemical Society,
Washington, DC, 1992, chap. 19; k) T. Iwamoto in Inclusion Com-
pounds, Vol. 5 (Eds.: J. L. Atwood, J. Davies, D. D. MacNicol), Oxford
University Press, New York, 1991, p.177.