Microsoft word - discography.doc
Patient Education Handout LUMBAR DISCOGRAPHY (Disc Stimulation) The discs of the spine may develop tears in the outside lining that holds the spongy, shock absorber part of the disc inside. The disc may then become painful causing pain due to nerves that grow into the disc where there should be none. Movement then causes severe back pain due to these new nerves being compressed.
 only 0.04–0.08 (Evans and Cameron, ’86). Thus,NH3 lipid solubility is only moderate and muchlower than that of CO2 (Knepper et al., ’89). As thelipid solubility of NH3 is not especially high, doesNH3 enter the lipid bilayer at all during its transitacross the gill epithelium?
As pointed out by Wood (’93), one possibility is
that NH3 moves through aqueous pores, ratherthan the lipid bilayers. Since the solubility inwater of NH3 is approximately 1,000 times greaterthan that of CO2 and is more than 20,000 timesgreater than that of O2 (Cameron and Heisler, ’83;Boutilier et al., ’84), it should readily move downfavourable PNH
through aquaporin 1 (AQP1) expressed in oocytes
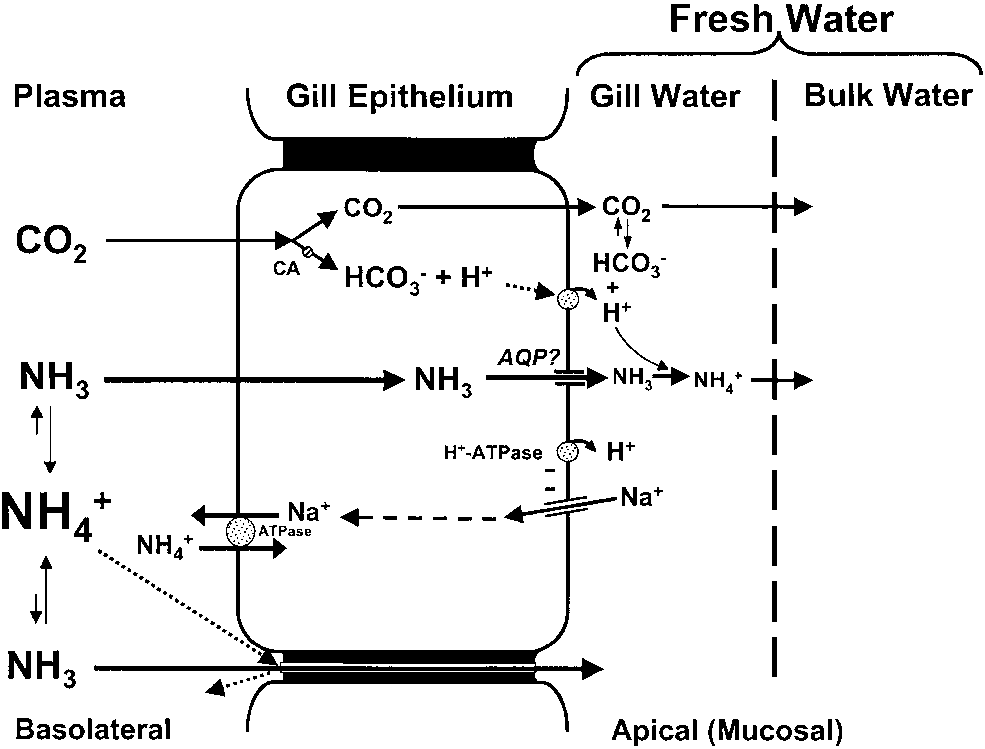
Model of ammonia excretion for fresh water fishes.
only 0.04–0.08 (Evans and Cameron, ’86). Thus,NH3 lipid solubility is only moderate and muchlower than that of CO2 (Knepper et al., ’89). As thelipid solubility of NH3 is not especially high, doesNH3 enter the lipid bilayer at all during its transitacross the gill epithelium?
As pointed out by Wood (’93), one possibility is
that NH3 moves through aqueous pores, ratherthan the lipid bilayers. Since the solubility inwater of NH3 is approximately 1,000 times greaterthan that of CO2 and is more than 20,000 timesgreater than that of O2 (Cameron and Heisler, ’83;Boutilier et al., ’84), it should readily move downfavourable PNH
through aquaporin 1 (AQP1) expressed in oocytes
Model of ammonia excretion for fresh water fishes. evidence that a V-type H+-ATPase is present in
accessory cells (Fig. 2; Sardet, ’80). Although
the apical epithelium of gill pavement cells (Lin
this arrangement substantially increases bran-
et al., ’94; Sullivan et al., ’95, ’96) suggests this
chial cation (Na+) permeability (Marshall, ’95;
transporter also contributes to gill water acidifica-
Karnaky, ’98), it is also likely that it provides a
tion (Lin and Randall, ’90). Indeed, as this H+-
ATPase is closely coupled to channel-mediated
Na+ uptake across the gills, it may explain why
Recently, a cultured branchial epithelial cell
the addition of the Na+ channel blocker amiloride
preparation comprised of both chloride cells and
to water inhibits JAmm (e.g., Kirschner et al., ’73;
pavement cells, and containing high-resistance
Payan, ’78; Wright and Wood, ’85; Yesaki and
‘‘tight junctions,’’ exhibited significant NH+
Iwama, ’92; Wilson et al, ’94; McGeer and Eddy,
NH3 permeance under fresh water conditions
’98). In such situations, amiloride would not only
interfere with Na+ channel access, it would alter
significantly correlated with the basolateral-to-
apical membrane potential and therefore inhibit
electrogenic H+-ATPase activity (Harvey, ’92;
across the preparation. Significant basolateral-to-
apical NH4 diffusion was also supported by the
or moderately buffered waters following amiloride
tight relationship between JAmm and the membra-
treatment likely reflects decreased boundary layer
ne’s electrical conductance, after correcting for
acidification resulting from decreased H+-ATPase
NH3 diffusion. Although convincing, it is still
mediated H+ extrusion. Indeed, when boundary
unclear how closely this preparation mimics the
layer acidification is impossible in highly buffered
true ‘‘in vivo’’ situation as the ammonia concen-
waters, amiloride has no affect on JAmm by
trations on the basolateral side of the preparation
rainbow trout, even in the face of large reductions
were relatively high (650 mmol Á LÀ1). Further,
(B90%) in Na+ uptake (Wilson et al., ’94).
evidence that a V-type H+-ATPase is present in
accessory cells (Fig. 2; Sardet, ’80). Although
the apical epithelium of gill pavement cells (Lin
this arrangement substantially increases bran-
et al., ’94; Sullivan et al., ’95, ’96) suggests this
chial cation (Na+) permeability (Marshall, ’95;
transporter also contributes to gill water acidifica-
Karnaky, ’98), it is also likely that it provides a
tion (Lin and Randall, ’90). Indeed, as this H+-
ATPase is closely coupled to channel-mediated
Na+ uptake across the gills, it may explain why
Recently, a cultured branchial epithelial cell
the addition of the Na+ channel blocker amiloride
preparation comprised of both chloride cells and
to water inhibits JAmm (e.g., Kirschner et al., ’73;
pavement cells, and containing high-resistance
Payan, ’78; Wright and Wood, ’85; Yesaki and
‘‘tight junctions,’’ exhibited significant NH+
Iwama, ’92; Wilson et al, ’94; McGeer and Eddy,
NH3 permeance under fresh water conditions
’98). In such situations, amiloride would not only
interfere with Na+ channel access, it would alter
significantly correlated with the basolateral-to-
apical membrane potential and therefore inhibit
electrogenic H+-ATPase activity (Harvey, ’92;
across the preparation. Significant basolateral-to-
apical NH4 diffusion was also supported by the
or moderately buffered waters following amiloride
tight relationship between JAmm and the membra-
treatment likely reflects decreased boundary layer
ne’s electrical conductance, after correcting for
acidification resulting from decreased H+-ATPase
NH3 diffusion. Although convincing, it is still
mediated H+ extrusion. Indeed, when boundary
unclear how closely this preparation mimics the
layer acidification is impossible in highly buffered
true ‘‘in vivo’’ situation as the ammonia concen-
waters, amiloride has no affect on JAmm by
trations on the basolateral side of the preparation
rainbow trout, even in the face of large reductions
were relatively high (650 mmol Á LÀ1). Further,
(B90%) in Na+ uptake (Wilson et al., ’94).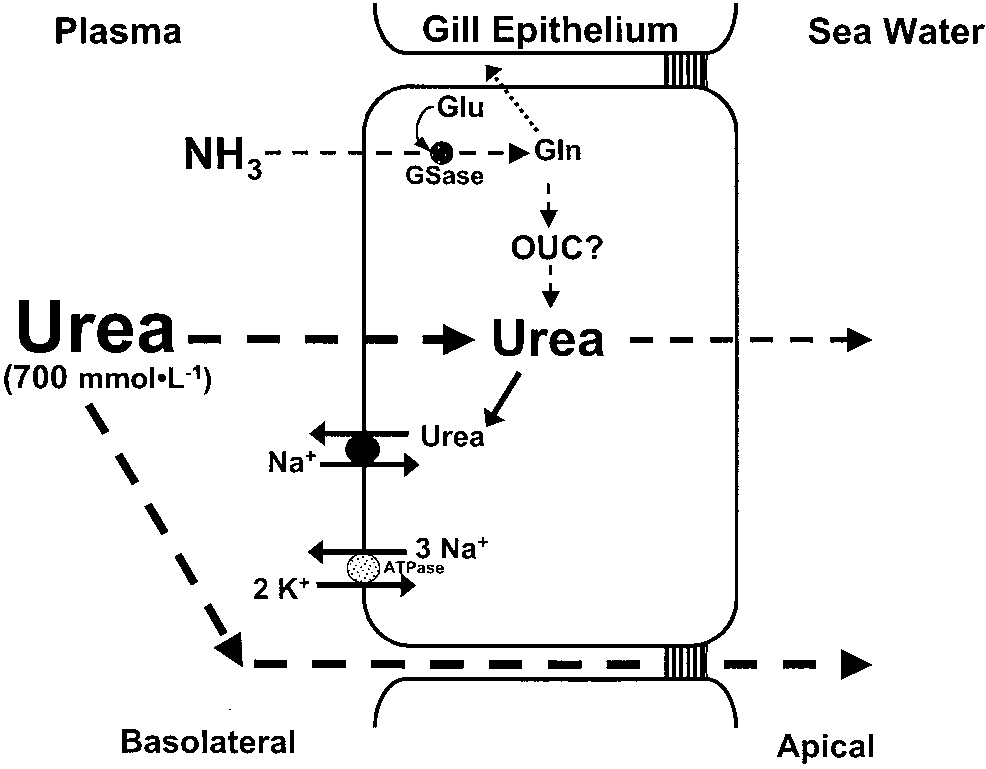 observations that small elevations in blood urea(15%) resulted in no change in JUrea in dogfish(Wood et al., ’95a). Further, acetamide andthiourea infusions led to increased branchial ureaclearance suggesting that these urea analogueswere competing with urea for binding sites on the‘‘back-transporter.’’ As basolateral application ofphloretin resulted in 2-fold increases in JUreaacross the gills of dogfish IPHPs, it lent furthersupport to the possible presence of an inwardlydirected, basolateral urea transporter (Pa
’98). Interestingly, these observations also ruledout a common route of urea and water movement,as phloretin had no effect on branchial water fluxmeasured using 3H2O. Further evidence favouringa basolateral versus apical location for the ureatransporter was provided by the much higher (14-
Model of ammonia and urea handling by the
fold) rates of urea efflux to the perfusion medium
elasmobranch gill. Although ammonia may be excreted in a
(basolateral side) versus the water (apical side)
manner that is similar to other marine fishes, branchial NH3losses may be minimized by the presence of glutamine
that resulted when urea was removed from each
synthetase in the gill cytosol, which promotes glutamine
formation from NH3 and glutamate. The resulting glutamine
could be exported to the liver, where it enters the ornithine
kidney, Smith and Wright (’99) first identified a
urea cycle (OUC), and/or be retained in the gill cytosol for
homologue to this protein in the elasmobranch gill
intra-branchial urea synthesis. A high cholesterol:phospholi-pid ratio in the basolateral membrane, along with a urea back-
using Northern analysis, but it is unlikely that
transporter(s), minimizes passive urea leakage across the gill.
observations that small elevations in blood urea(15%) resulted in no change in JUrea in dogfish(Wood et al., ’95a). Further, acetamide andthiourea infusions led to increased branchial ureaclearance suggesting that these urea analogueswere competing with urea for binding sites on the‘‘back-transporter.’’ As basolateral application ofphloretin resulted in 2-fold increases in JUreaacross the gills of dogfish IPHPs, it lent furthersupport to the possible presence of an inwardlydirected, basolateral urea transporter (Pa
’98). Interestingly, these observations also ruledout a common route of urea and water movement,as phloretin had no effect on branchial water fluxmeasured using 3H2O. Further evidence favouringa basolateral versus apical location for the ureatransporter was provided by the much higher (14-
Model of ammonia and urea handling by the
fold) rates of urea efflux to the perfusion medium
elasmobranch gill. Although ammonia may be excreted in a
(basolateral side) versus the water (apical side)
manner that is similar to other marine fishes, branchial NH3losses may be minimized by the presence of glutamine
that resulted when urea was removed from each
synthetase in the gill cytosol, which promotes glutamine
formation from NH3 and glutamate. The resulting glutamine
could be exported to the liver, where it enters the ornithine
kidney, Smith and Wright (’99) first identified a
urea cycle (OUC), and/or be retained in the gill cytosol for
homologue to this protein in the elasmobranch gill
intra-branchial urea synthesis. A high cholesterol:phospholi-pid ratio in the basolateral membrane, along with a urea back-
using Northern analysis, but it is unlikely that
transporter(s), minimizes passive urea leakage across the gill.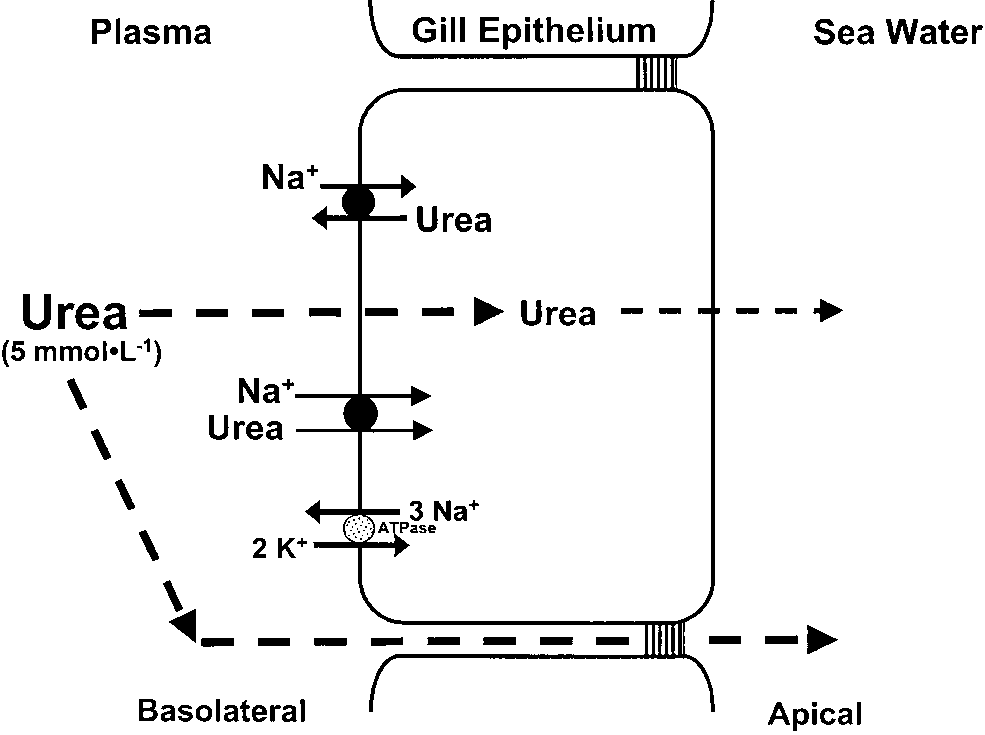 ¨rt et al. (’99) suggested that the very low
branchial urea permeabilities seen in non-pulsingtoadfish (10À8 cm Á secÀ1) may actually representthe ‘‘true’’ diffusive permeabilities of teleost gills,and that higher branchial urea permeabilities inother fishes (B10À6 cm Á secÀ1) reflect the presenceof moderate numbers of facilitated urea transpor-ters. The recent cloning of a cDNA for an eel(Anguilla japonica) urea transporter (eUT; Mistryet al., 2001) appears to substantiate this hypoth-esis. However, it is not clear if this urea transpor-ter is involved in urea excretion or retention.
¨rt et al. (’99) suggested that the very low
branchial urea permeabilities seen in non-pulsingtoadfish (10À8 cm Á secÀ1) may actually representthe ‘‘true’’ diffusive permeabilities of teleost gills,and that higher branchial urea permeabilities inother fishes (B10À6 cm Á secÀ1) reflect the presenceof moderate numbers of facilitated urea transpor-ters. The recent cloning of a cDNA for an eel(Anguilla japonica) urea transporter (eUT; Mistryet al., 2001) appears to substantiate this hypoth-esis. However, it is not clear if this urea transpor-ter is involved in urea excretion or retention.